LivaNova’s recall of 484 LifeSPARC circulatory support systems from the U.S. has been labelled as a Class I event by the Food and Drug Administration. LivaNova alerted customers in July to a software malfunction that can result in the pump stopping for an extended period of time. The FDA concluded the fault may cause serious injuries or death, leading it to assign the recall to its highest risk category. To date, LivaNova has received 66 complaints about the fault. The FDA has received reports of two injuries and no deaths.
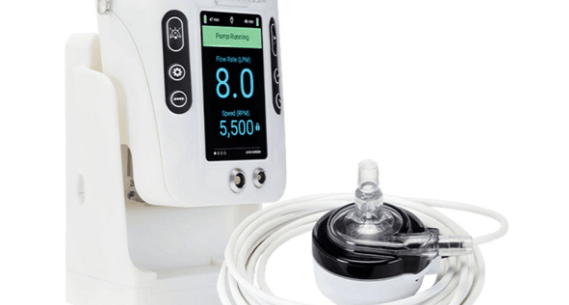
LifeSPARC circulatory support systems are designed to pump blood for up to six hours during open-heart surgery. The device features two components, a single-use pump and a controller that serves as the user interface and provides power and electrical signals.
The recall covers the LifeSPARC Controller. Because of a fault, the controller may mistakenly detect frozen or unresponsive software and put the device into critical failure mode, according to the FDA. The mode clears the controller screen and causes an alarm that cannot be muted or turned off.
While the pump should continue to run at the set speed in critical failure mode, the need to replace the controller can cause problems. The user needs to follow specific instructions for replacing the controller and power the frozen device off before acquiring and setting up the backup controller. If the user fails to perform those steps, the pump may stop for an extended period of time while the controller is replaced, the agency said.
In light of the risk, LivaNova asked customers to confirm that the pump is operating at the set speed if the screen freezes. Users should continue to control the speed using the up and down arrows on the controller and follow the instructions in the operations manual when replacing the controller, according to the FDA. Sites that use the device should ensure a backup controller is available.