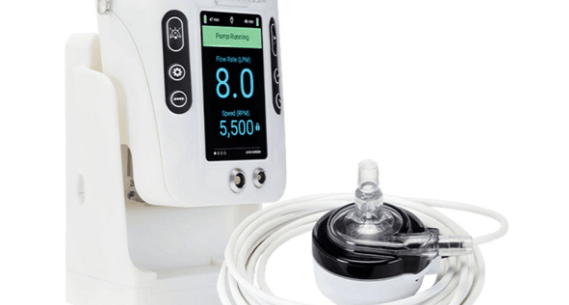
Liva Nova Recall Of Open-Heart-Surgery Blood Pump Designated As Class I Event By FDA
LivaNova’s recall of 484 LifeSPARC circulatory support systems from the U.S. has been labelled as a Class I event by the Food and Drug Administration. LivaNova alerted customers in July…